Facility and Process Overview
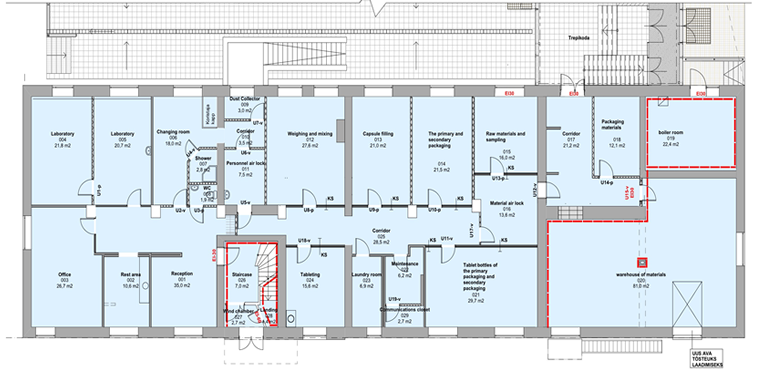
•GPE GLOBAL's Facility is licensed by the European Union through the Estonian State Agency of Medicine (SAM) and has qualified for Good Manufacturing Process (GMP) status for its packaging operations.•The facility Occupies 505 square meters of GMP-compliant space in the Tartu Biotechnology Park, an innovative business incubator for biotechnological, veterinary and medicine based enterprises.
•Production facility contains hard capsule filling and tablet manufacturing lines of pharmaceutical manufacturing. Packaging space is designed for primary and secondary clinical packaging and includes both bottling and PVC-foil blister-packaging equipment.